This website is best viewed using the vertical display on your mobile device.
- SPRYCEL Patient Site
- BMS Resources
- Full Prescribing Information
- Indications
INDICATIONS
SPRYCEL® (dasatinib) is indicated for the treatment of adult patients with:
- Newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase
- Chronic, accelerated, or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including imatinib
- Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance or intolerance to prior therapy
SPRYCEL® is indicated for the treatment of pediatric patients 1 year of age and older with:
- Newly diagnosed Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) in combination with chemotherapy
- Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase
SPRYCEL patients achieved higher & faster cCCyR* and MMR† rates at Years 1 and 5 vs imatinib1‡
- Results based on a Phase III, open-label, randomized study of SPRYCEL® (dasatinib) 100 mg (n=259) once daily vs imatinib 400 mg once daily (n=260) in adults with newly diagnosed CP Ph+ CML (N=519)1,2
- Primary endpoint was the rate of cCCyR by 12 months1,2
- Select secondary endpoints included: MMR at any time, time to MMR, and time to cCCyR1,2
- cCCyR* RatesYear 1 & 5 Data
- MMR† RatesYear 1 & 5 Data
- Transformation Rates


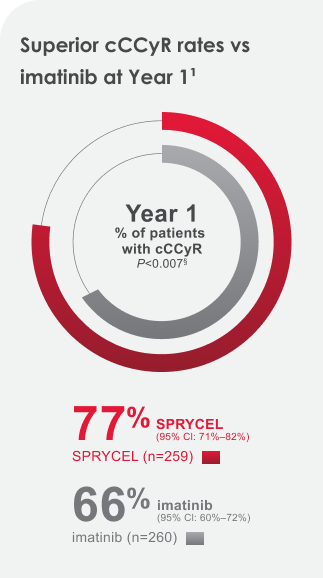


77% of SPRYCEL patients achieved the cCCyR 12-month treatment milestone. 83% achieved the Year 5 treatment milestone.1
In newly diagnosed chronic phase CML patients:
- Drug-related serious adverse reactions (SARs) were reported for 16.7% of SPRYCEL-treated patients. Serious adverse reactions reported in ≥5% of patients included pleural effusion (5%)
- The most common adverse reactions (≥15%) include myelosuppression, fluid retention, and diarrhea


Achieving MMR is important on a patient’s treatment path5




IMPORTANT INFORMATION ABOUT ADVERSE REACTIONS
- With a minimum of 60 months follow-up, adverse reactions that were reported in
≥10% of patients with newly diagnosed chronic phase CML for patients taking
SPRYCEL (n=258) included:
- All grades: fluid retention (38%) [including pleural effusion (28%), superficial localized edema (14%), pulmonary hypertension (5%), generalized edema (4%), pericardial effusion (4%), congestive heart failure/cardiac dysfunction|| (2%), pulmonary edema (1%)], diarrhea (22%), musculoskeletal pain (14%), rash¶ (14%), headache (14%), abdominal pain (11%), fatigue (11%), nausea (10%), myalgia (7%), arthralgia (7%), hemorrhage# (8%) [including gastrointestinal bleeding (2%), other bleeding** (6%)], vomiting (5%), muscle spasms (5%)
- Grade 3/4: fluid retention (5%) [including pleural effusion (3%), pulmonary hypertension (1%), pericardial effusion (1%)], diarrhea (1%), hemorrhage (1%) [including gastrointestinal bleeding (1%)]


After 5 years of follow-up, median time to MMR among responders was 9.3 months in the SPRYCEL arm (n=198) and 15 months in the imatinib arm (n=167)1


Baseline Hasford risk scores1,2
- SPRYCEL: low: 33% (n=86);
intermediate: 48% (n=124);
high: 19% (n=49) - imatinib: low: 34% (n=87);
intermediate: 47% (n=123); high: 19% (n=50)
Patient transformations by year while on study9


SPRYCEL patients experienced no new transformations after Year 3 and continuing through Year 59


Imatinib patients experienced 2 new transformations after Year 3 and continuing through Year 59
94% of imatinib-treated patients on study did not transform to accelerated phase or blast crisis (245/260)1,9
All randomized patients who transformed while on study progressed to either accelerated phase or blast crisis.1
- Lack of transformation to accelerated or blast phase is a key indicator of lack of disease progression10
IMPORTANT INFORMATION ABOUT MYELOSUPPRESSION
Treatment with SPRYCEL is associated with severe (NCI CTCAE Grade 3/4) thrombocytopenia, neutropenia, and anemia, which occur earlier and more frequently in patients with advanced phase CML or Ph+ ALL than in patients with chronic phase CML. Myelosuppression was reported in patients with normal baseline laboratory values as well as in patients with pre-existing laboratory abnormalities.
- In patients with chronic phase CML, perform complete blood counts (CBCs) every 2 weeks for 12 weeks, then every 3 months thereafter, or as clinically indicated
- Myelosuppression is generally reversible and usually managed by withholding
SPRYCEL temporarily and/or dose reduction
- In clinical studies, myelosuppression may have also been managed by discontinuation of study therapy
- Hematopoietic growth factor has been used in patients with resistant myelosuppression
Dasatinib does not appear to be active against the T315I mutation, based on in vitro data.1
BCR-ABL=breakpoint cluster region-Abelson kinase; cCCyR=confirmed complete cytogenetic response; CI=confidence interval; CP=chronic phase; MMR=major molecular response; Ph+ CML=Philadelphia chromosome-positive chronic myeloid leukemia; RQ-PCR=real-time quantitative polymerase chain reaction.
*cCCyR was defined as CCyR (0% Ph+ metaphases) on 2 consecutive occasions at least 28 days apart.1,2
†MMR (at any time) defined as BCR-ABL ratios of ≤0.1% by RQ-PCR in peripheral blood samples standardized on the International Scale. These are cumulative rates representing minimum follow-up for the time frame specified.1,2
‡Inclusive of cCCyR in newly diagnosed adult patients with CP Ph+ CML by 1 and 5 years.1
§Adjusted for Hasford score and indicated statistical significance at a predefined nominal level of significance.1
||Includes cardiac failure acute, cardiac failure congestive, cardiomyopathy, diastolic dysfunction, ejection fraction decreased, and left ventricular dysfunction.1
¶Includes erythema, erythema multiforme, rash, rash generalized, rash macular, rash papular, rash pustular, skin exfoliation, and rash vesicular.1
#Adverse reaction of special interest with <10% frequency.1
**Includes conjunctival hemorrhage, ear hemorrhage, ecchymosis, epistaxis, eye hemorrhage, gingival bleeding, hematoma, hematuria, hemoptysis, intra-abdominal hematoma, petechiae, scleral hemorrhage, uterine hemorrhage, and vaginal hemorrhage.1
resistant or intolerant to imatinib
References:
- SPRYCEL full Prescribing Information. Bristol-Myers Squibb Company.
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260-2270.
- Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809-1820.
- Kantarjian H, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119(5):1123-1129.
- Cortes J, Quintas-Cardama A, Kantarjian HM. Monitoring molecular response in chronic myeloid leukemia. Cancer. 2011;117(6):1113-1122.
- Jabbour E, Kantarjian H, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123(4):494-500.
- Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333-2340.
- Data on file. SPRY 064. Bristol-Myers Squibb Company; 2016.
- Data on file. SPRY 061. Bristol-Myers Squibb Company; 2016.
- Bavaro L, Martelli M, Cavo M, Soverini S. Mechanism of disease progression and resistance to tyrosine kinase inhibitor therapy in chronic myeloid leukemia: an update. Int J Mol Sci. 2019;20(24):6141.





